Other Immunomodulatory And Cytotoxic Agents
Some additional immunomodulatory drugs are used in RA including azathioprine , and cyclosporin A . Rarely cyclophosphamide and d-Penicillamine are used. Because the potential of high toxicity, these agents are typically utilized for life-threatening extra-articular manifestations of RA such as systemic vasculitis or with severe articular disease that is refractory to other therapy.
Azathioprine has some activity in rheumatoid arthritis but may take 8-12 weeks to see an effect. It is a purine analog that can cause bone marrow suppression and lowering of blood cell counts particularly in patients with renal insufficiency or when used concomitantly with allopurinol or ACE inhibitors. Increased risk of secondary malignancy due to azathioprine is controversial. Screening for levels of the enzyme thiopurine methyltransferase is recommended before initiating therapy with azathioprine. Certain individuals have deficiencies in this enzyme that metabolizes azathioprine with a concomitantly increased risk of toxicitiy for the medication. Side effects include nausea, and alopecia. Blood tests to monitor blood counts and liver function tests are necessary for patients on azathioprine.
How Many Capsules Should I Take
For daily use, we recommend taking 1 capsule so that the natural ingredients can provide the optimal environment for your body to heal your joints.
For situational use in response to particularly painful joints, we recommend taking 2 to 4 capsules as needed for maximum strength relief.
Proper dosage is an individual process that depends on what works best for you and your body. We do not recommend taking more than 6 capsules per day.
Newer Rheumatoid Arthritis Drug May Help Ease Tough
HealthDay Reporter
MONDAY, Oct. 19, 2020 — A recently approved rheumatoid arthritis medication appears to be an effective second-line therapy when biologic treatments start to fail, a new clinical trial reports.
Arthritis sufferers treated with upadacitinib had a significantly greater reduction in their symptoms and disease activity than people treated with a standard disease-modifying antirheumatic drug , said co-researcher Dr. Aileen Pangan. She is executive medical director of immunology clinical development for the pharmaceutical company AbbVie in North Chicago, Ill.
The drug, marketed under the brand name Rinvoq, also helped twice as many patients enter remission from their rheumatoid arthritis, according to a report in the Oct. 15 issue of the New England Journal of Medicine.
“Upadacitinib has shown superiority to one of the current standard-of-care treatment options in the clinic for these difficult-to-treat patients,” Pangan said. “It is important for physicians to have multiple treatment options available, including medications with different mechanisms of action, to help provide patients with the treatment that is right for them.”
Rinvoq received U.S. Food and Drug Administration approval in August 2019 for treatment of moderate to severe rheumatoid arthritis.
This 24-week clinical trial aimed to assess Rinvoq’s effectiveness in helping rheumatoid arthritis patients for whom DMARD treatment had failed.
Read Also: Rheumatoid Arthritis Pain Description
Popular Heart Surgery Carried Hidden Danger
One difficulty in assessing whether arthritis drugs cause cardiovascular problems is that the disease itself is a risk factor for heart disease. Thats where longer-term and larger post-marketing safety studies can help.
In one of these studies, required by the FDA, Actemra was compared head-to-head with another arthritis drug Enbrel, whose label strongly cautions about use by patients with cardiovascular disease, particularly heart failure. That was the study Siegel called definitive evidence of Actemras safety.
It found rates of stroke and heart failure were about 1.5 times higher in Actemra patients. That difference wasnt statistically significant, but experts told STAT it was still worrisome.
Since Enbrel includes a high-profile warning and precaution in the label about heart failure, it is concerning that Actemra might be similar or worse, said Dr. Steven Woloshin, a professor of medicine at The Dartmouth Institute and an expert on risk communication.
Felson concurred. While the actual risk remains statistically uncertain, he said, if Enbrels label warns for heart failure, the FDA should consider that warning for Actemra.
If the rates are similar, it should be on the warning label, although the company and FDA might have additional data to consider, Pisetsky said.
That hasnt happened.
Steroid Use For Rheumatoid Arthritis Treatment Should Be Minimized
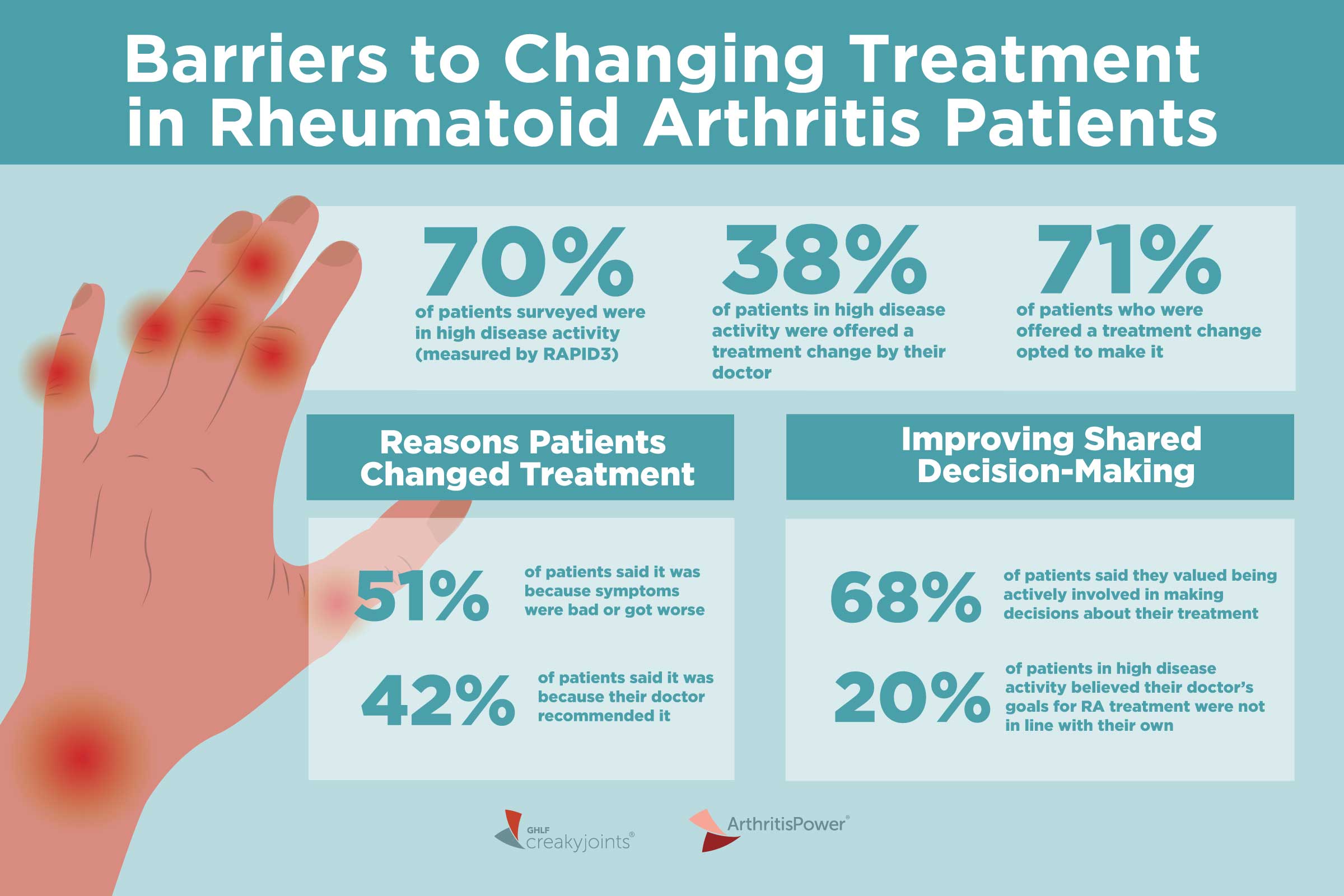
One important shift from prior guidance is an emphasis that glucocorticoids, or steroids, should be prescribed as infrequently as possible.
Steroids are often used as a bridge therapy when a patient is first diagnosed, before their DMARD has time to work. But steroids come with many risks, including infection, weight gain, bone fractures, and osteoporosis. Because of these serious side effects, the guideline recommends that doctors curtail their use.
The panel acknowledged that steroids are often necessary to act as this bridge, but that their toxicity was judged to outweigh potential benefits,” and therefore they should be limited to the lowest effective dose for the shortest duration possible.
At the 2020 press conference, Dr. Fraenkel emphasized that “doctors should try to push the needle away from using prednisone as frequently as we do.
RELATED: Home Remedies and Alternative Treatments for Rheumatoid Arthritis
Read Also: How To Ease Arthritis Pain In Fingers
Glucosamine Is Doing More Harm Than Good
A 6 month placebo-controlled trial was ended early since glucosamine resulted in worse joint pain.*
In a with 164 participants, subjects taking glucosamine and chondroitin reported significantly worse pain according to the WOMAC scale, which measures joint pain, stiffness, and physical function. This led the doctors to end the trial early in the interest of the subjects’ health.
But if glucosamine is so harmful, why do 20% of adults still take it?
The answer lies in the placebo effect. When people take action to address a problem, even simply swallowing a pill can convince your mind that your body feels better.
Three Categories Of Drugs
The formula for treating RA often is a mix. Doctors draw from three main groups of FDA-approved medicines:
1. Nonsteroidal anti-inflammatory drugs ease pain and inflammation. Some, like ibuprofen and naproxen sodium, are over-the-counter drugs. You need a prescription for others, including a kind called COX-2 inhibitors, which can be easier on your stomach.
2. Corticosteroids, including prednisone, act quickly to control inflammation. These strong drugs have strong side effects, so doctors limit the dose and how long you take them.
3.Disease-modifying antirheumatic drugs can alter the course of RA and prevent joint and tissue damage. They block the effects of chemicals released when your immune system mistakenly attacks your joints. Methotrexate is usually the first DMARD prescribed, often as soon as someone is diagnosed. Doctors now know that a delay might make your RA worse.
Also Check: How Do I Know If I Have Knee Arthritis
What Is Rheumatoid Arthritis
Arthritis is a general term that describes inflammation in joints. Rheumatoid arthritis is a type of chronic arthritis that occurs generally in joints symmetrically . This involvement of several joints helps distinguish rheumatoid arthritis from other types of arthritis.
In addition to affecting the joints, rheumatoid arthritis may occasionally affect the skin, eyes, lungs, heart, blood, nerves or kidneys.
Will There Ever Be A Cure
Currently, there is no cure for RA. Doctors use the latest treatments to help people with RA manage their symptoms and prevent the disease from progressing.
When asked about the possibility of a cure, Dr. Koval indicated a positive outlook. Seeing how far we have come with RA in just the 30 years, it makes me encouraged that we will one day find a cure. Science and technology advanced considerably in this field, and I am excited at what the future may bring.
Dr. Martinez felt more cautiously optimistic. It is not clear if or when we will have a cure for RA, he stated. There is significant research going on to better help us understand the causes and mechanisms that lead to RA, perhaps helping us reach that lofty goal in the future.
Even without a cure on the immediate horizon, it is possible to achieve
Read Also: How To Treat Lower Back Arthritis
First Update To Ra Treatment Guidelines In 5 Years
The recommendations update previous guidelines from 2015, continuing the ACRs practice of reevaluating and revamping its guidelines every five years, according to Liana Fraenkel, MD, MPH, an adjunct professor of medicine at Yale University School of Medicine in New Haven, Connecticut, and the lead investigator for the update.
RELATED: Arthritis Lifestyle Types: Which One Are You?
Good Alternative For People Who Dont Respond Well To Methotrexate
Produced by AbbVie, the drug is in a class called Janus kinase inhibitors, which help with pain and swelling by suppressing overactive immune systems in people with RA. For patients who dont respond or only respond partially to methotrexate, this is a really important development, says Jonathan Krant, MD, chief of rheumatology at Marietta Health Systems in Ohio, and professor of medicine at Ohio University in Athens.
Roy M. Fleischmann, MD, a primary investigator for SELECT, the name of the clinical study program, and clinical professor at the University of Texas Southwestern Medical Center in Dallas, said in an AbbVie press release from August 16, 2019, “Despite the availability of multiple treatment options with varying mechanisms of action, many patients still do not achieve clinical remission or low disease activity the primary treatment goals for rheumatoid arthritis. With this FDA approval, Rinvoq has the potential to help additional people living with RA achieve remission who have not yet reached this goal.”
Related: Rheumatoid Arthritis Treatment: Is Your Doctor Using Treat-to-Target Protocols?
Read Also: How To Ease Arthritis Pain In Fingers
The Move From Step Therapy Is In Response To Patients And Patient Advocates
This recommendation was driven by patients, Fraenkel said at the press conference. Patients who are suffering want to move ahead with a biologic so they can quickly feel better. What physicians consider a short amount of time, patients do not, she said.
RELATED: A Laugh-Out-Loud Author Takes on a Deadly Serious Topic: Fighting for Health Insurance Coverage
Dr. Thomas notes that patients also dislike the intense regimen of the medicines and also the many tests, from blood labs to eye exams, they must regularly undertake on triple therapy. As a clinician, he said at the press conference, Im so thankful for this recommendation.
In some cases, insurance companies require patients to try and fail triple therapy before moving to a biologic. Dr. Ludmer feels strongly that different patients have different needs, and that doctors know each individual patient best. Insurers dont always allow for individualizing a treatment. Cost is a factor, but the decision of what drugs are best for patients should be data driven, not insurance driven, she says.
What Drugs Are Used To Treat Rheumatoid Arthritis
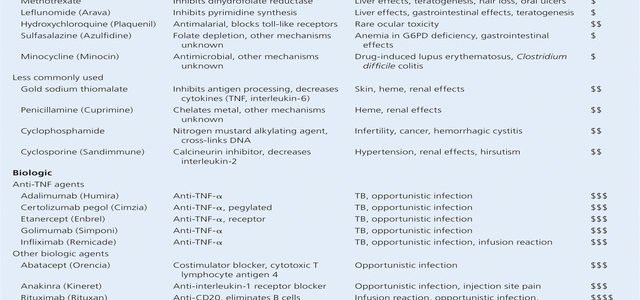
The drugs used to treat rheumatoid arthritis can be divided into three groups:
- Drugs that decrease pain and inflammation. These products include non-steroidal anti-inflammatory drugs , such as ibuprofen , naproxen , and other similar products. Another type of drug the COX-2 inhibitor also falls into this drug category, providing relief of the signs and symptoms of rheumatoid arthritis. Celecoxib , one COX-2 inhibitor, is available and used in the United States. The COX- 2 inhibitors were designed to have fewer bleeding side effects on the stomach.
- Disease-modifying antirheumatic drugs . Unlike other NSAIDs, DMARDs can actually slow the disease process by modifying the immune system. Older DMARDs include methotrexate , gold salts, penicillamine , hydroxychloroquine , sulfasalazine , cyclosporine , cyclophosphamide and leflunomide . Currently, methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine are the most commonly used. Many of these drugs were first used to treat other medical conditions such as malaria, transplant rejection, cancer, psoriasis and inflammatory bowel disease but have now also found a role in treating rheumatoid arthritis.
Recommended Reading: How Do You Know You Have Arthritis
What Are The Latest Approved Treatments For Rheumatoid Arthritis
There are several new Rheumatoid Arthritis medications that have been approved and others that remain under clinical trial. Here are some of them:
Jyseleca 4
Jyseleca is a Janus kinase 1 inhibitor indicated for the treatment of adults with moderate to severe active rheumatoid arthritis .It is indicated for patients who do not respond or are intolerant to other anti-rheumatic medicines. It can be used on its own or together with another anti-rheumatic medicine, methotrexate .
Jyseleca was approved for the acute treatment of adults with migraine by the European Medicines Agency , Europe, on September 24, 2020 and by the Pharmaceuticals and Medical Devices Agency , Japan, on September 25, 2020.
Rinvoq 5,6
Rinvoq is an oral, once daily selective and reversible JAK inhibitor used for the expanded use in two additional rheumatic indications: the treatment of adult patients with active psoriatic arthritis and adult patients with active ankylosing spondylitis. It is used for moderate or severe rheumatoid arthritis that cannot be controlled well enough with disease-modifying anti-rheumatic medicines or if the patient cannot take these medicines.
Rinvoq was approved for the treatment of moderate or severe Rheumatoid Arthritis by the by the Food and Drug Administration , US, on Aug 16, 2019 and by the European Medicines Agency , Europe, on Dec 12, 2019.
Otilimab7
Otilimab has not been approved anywhere in the world.
Therapy Is Not Always The Best Approach For Successful Ra Care
In the 2015 guidelines, doctors were advised to escalate to triple therapy a combination of methotrexate, sulfasalazine, and hydroxychloroquine before starting a biologic in patients whose disease was uncontrolled. The new guidelines reverse this, recommending that rheumatologists add a biologic or targeted synthetic DMARD instead of switching to triple therapy.
According to the new guidelines, consensus on this recommendation was not as easily achieved as for most others. The decision on whether patients with an inadequate response to methotrexate should escalate to a DMARD, DMARD, or triple therapy engendered much discussion with contrasting points of view, the researchers wrote.
Ultimately, the recommendation to bypass triple therapy was made because of the more rapid onset of benefit and concerns related to the poor tolerability and durability of triple therapy in real-world practice.
Recommended Reading: What Does The Rash From Psoriatic Arthritis Look Like
Do I Have To Sign Up For Auto
Absolutely not! Auto-Delivery is optional for all of our products.
If you order RediMove every month, we recommend that you customize your own Auto-Delivery plan for your own convenience. All Auto-Delivery shipments come with free shipping and a full 30 day money back guarantee, no matter how many bottles you order. However, it is not required.
What Is The Biological Cause Of Joint Pain
A little-known signaling molecule is the key to reducing inflammation.
Research reveals that highly active senior adults have a unique biological advantage that limits the production of a specific type of inflammatory molecule and protects their joints from wear and tear.
Continual wear and tear results in a buildup of pro-inflammatory cytokines, which keeps your joints inflamed.
Limiting the production of pro-inflammatory cytokines reduces inflammation, creating the optimal environment to regenerate damaged cartilage. This allows you to move pain free again and return to an active lifestyle.
You May Like: Arthritis Remission
Rheumatoid Arthritis Treatment Has Advanced Significantly In Recent Years
RA treatments have several different goals, according to the ACR: reduce symptoms, including pain prevent joint damage enhance a patients function and quality of life and lessen complications of the disease.
The primary treatment for RA has long centered on synthetic disease-modifying antirheumatic drugs, known as DMARDs. This includes older drugs like methotrexate and sulfasalazine as well as newer biologics and JAK inhibitors.
All these medicines, along with quality testing and other management tools, have transformed the treatment of RA, said Don Thomas, MD, a board-certified rheumatologist in Greenbelt, Maryland, at a press conference in 2020 announcing the draft report. While years ago, many of his RA patients were debilitated and in wheelchairs, today most are in remission or experience low levels of disease.
New Treatment For Rheumatoid Arthritis Granted Fda Approval
Officials with the FDA have approved AbbVies upadacitinib 15 mg, once-daily oral Janus kinase inhibitor, for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate .
Officials with the FDA have approved AbbVies upadacitinib 15 mg, once-daily oral Janus kinase inhibitor, for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate .
This new therapy is expected to be available in the United States later this month.
Designed to help accommodate people living with RA, AbbVies packaging for upadacitinib includes a bottle cap with a wide, easy-to-grip texture and an embedded tool that punctures the foil liner to simplify medication access.
“Rheumatoid arthritis can have a debilitating impact on the lives of those with the chronic disease, including making it difficult to perform everyday tasks,” said Cindy McDaniel, senior vice president, consumer health, Arthritis Foundation, in a prepared statement.
Across the SELECT Phase 3 studies, RINVOQ met all primary and ranked secondary endpoints. The primary endpoints include:
Reference
Related Content:
Also Check: Arthritic Pain Symptoms
Evidence For Effective Drug Tapering Is Low
The panel noted that people who stop their medications risk flares and potential long-term joint damage.
For this reason, they recommend that people stay on at least one DMARD at a therapeutic dose.
RELATED: Stress and Rheumatoid Arthritis: News You Can Use From EULAR 2021
Those who have reached their target for at least six months may attempt to taper their medicine if they and their physician feel it is necessary.
Continuation of all DMARDs at their current dose is conditionally recommended over a dose reduction of a DMARD, dose reduction is conditionally recommended over gradual discontinuation of a DMARD, and gradual discontinuation is conditionally recommended over abrupt discontinuation of a DMARD for patients who are at target for at least 6 months, the guidelines state.