What You Need To Know Before You Take Otezla
Do not take Otezla:
- if you are allergic to apremilast or any of the other ingredients of this medicine .
- if you are pregnant or think you may be pregnant.
Warnings and precautions Depression and suicidal thoughtsSevere kidney problemsIf you are underweightGut problemsChildren and adolescentsOther medicines and Otezla
- rifampicin an antibiotic used for tuberculosis
- phenytoin, phenobarbital and carbamazepine medicines used in the treatment of seizures or epilepsy
- St Johns Wort a herbal medicine for mild anxiety and depression.
Pregnancy and breast-feeding Driving and using machinesOtezla contains lactose
Do You Need To Modify Psoriatic Arthritis Medications Before Or After Getting The Vaccine
Temporarily stopping certain immunosuppressant medications after receiving the vaccine, or timing when you get the vaccine in the course of your treatment, might help increase the effectiveness of the COVID-19 vaccine if you have PsA.
But this only applies to a select few psoriatic arthritis medications. It is recommended, in most cases, that PsA patients who are to receive a COVID-19 vaccine continue their biologic or oral therapies for psoriatic arthritis, says Dr. Gupta.
The American College of Rheumatology and National Psoriasis Foundation guidance differs on this matter, which is why its important to discuss this with your doctor and make a decision thats right for your situation.
Here are the psoriatic arthritis drugs for which the ACR guidance suggests changes may be recommended:
- Methotrexate: Skip for 1 week after each vaccine dose
- JAK inhibitor : Skip for 1 week after each vaccine dose
- Abatacept , injectable form: Skip one week before and after the first vaccine dose only
- Abatacept , IV form: Get COVID-19 vaccine 4 weeks after your last infusion, then skip a week and get next infusion
The NPF guidance recommends that patients continue their biologic or oral therapies for psoriatic arthritis in most cases. For the Johnson & Johnson COVID-19 vaccine, which is only one dose, the guidance says that certain patients can consider holding methotrexate for two weeks after getting the vaccine:
How Is Apremilast Taken
When you first start taking apremilast, youll be given a special starter pack which contains all the doses for the first six days. The pack is clearly labelled to make sure you take the right dose at the right time, starting with a low dose and working up to the maximum.
Once youve finished the starter pack, youll remain on the higher dose. Most people will take one tablet in the morning and one in the evening. However, if you have kidney problems, your specialist may suggest just taking one tablet a day.
If you miss a dose, contact your specialist team immediately for advice on when to take the next one.
Because apremilast is a long-term treatment, its important to keep taking it, unless you have severe side effects:
- even if it doesnt seem to be working at first
- even when your symptoms improve, to help keep your condition under control.
Your doctor may decide to stop the treatment after four months if there hasnt been enough improvement in your symptoms.
Read Also: Rheumatoid Arthritis In Arms
More Common Side Effects
The more common side effects of Otezla include:
- diarrhea
- weight loss
- back pain
Most of these effects may go away within a few days or a couple of weeks. If theyre more severe or dont go away, talk to your doctor or pharmacist.
* This is a partial list of mild side effects from Otezla. To learn about other mild side effects, talk with your doctor or pharmacist, or visit Otezlas prescribing information.
Otezla For Plaque Psoriasis
Otezla is approved to treat moderate to severe plaque psoriasis in adults. This is the most common form of psoriasis. Its characterized by thick red patches of skin that often have a silver or white scaly layer.
Effectiveness for plaque psoriasis
In clinical studies, about 30% of people taking Otezla had clearer skin and fewer plaques. In comparison, about 5% of people taking a placebo had that same result. For about 20% of people, their plaques cleared completely or almost completely. And 4% of people taking a placebo had the same result.
Also Check: What Is Cirrhotic Arthritis
Otezla For Psoriatic Arthritis
Otezla is approved to treat active psoriatic arthritis in adults. Psoriatic arthritis is a condition that has both the swollen, sore joints of arthritis and the skin lesions of psoriasis. Skin lesions caused by psoriasis are usually itchy, scaly red patches. In some cases, the lesions can affect your scalp.
Effectiveness for psoriatic arthritis
In clinical studies, Otezla improved symptoms of this condition by 20% in about 30% to 40% of people who took it. In comparison, 18% to 19% of people taking a placebo had that same result.
Are There Other Treatment Options For Psoriatic Arthritis
Yes. There are various medications available to treat the signs and symptoms of psoriatic arthritis. The medications range from anti-inflammatory meds such as ibuprofen, to disease modifying agents like methotrexate, to injectable biologics like Enbrel or Humira.
It is important to talk to your doctor and find out which option may be right for you.
Don’t Miss: Swollen Hot Finger Joint
Cost Insurance Coverage And Availability
Stelara and Otezla are both specialtydrugs, which are high-cost medications used to treat certain chronic conditions. Usually, only large specialty pharmacies stock specialty drugs.
Both of these drugs are expensive. However, at the time this article was written, the estimated monthly cost for Stelara was quite a bit higher than for Otezla .
Your insurance may not cover either of these drugs. Ask your pharmacist to check your insurance to see if it covers these drugs. If it doesnt, talk with your doctor about other payment options. For instance, the drugs manufacturers may offer programs that help cover the cost of the drugs.
Rated For Psoriatic Arthritis Report
Only 4 weeks in. My doctor recommended an extended ramp up to 30 mg twice a day. Basically did the first dose twice, second twice, etc. until I reached full dose. Side effects have been mild, a little diarrhea the first week now just urgency at times. I’m starting to feel relief in my feet and hands especially, I no longer have the constant pain that I had before. Still getting sharp pain at times with movement but only occasionally. Hoping that will be resolved as I continue treatment.
Also Check: Psoriatic Arthritis Hands Rash
How And When Does The Psoriatic Arthritis Usually Develop
Several factors play a role in the development of the arthritis such as your genetics, immune system, plus environmental factors. It is believed that 30% of psoriasis patients between the ages of 30 and 50 years old will go on to develop psoriatic arthritis. If you have been diagnosed with psoriasis, it is important to tell your dermatologist if you develop any aches or pains.
Safety Profile Of Apremilast
In PALACE 1 there was a low incidence of adverse events and most AEs were mild or moderate. Discontinuation in the trial due to AEs was 6.0% in the apremilast 20 mg group, 7.1% in the apremilast 30 mg group, and 4.8% in the placebo group. The only AEs reported in > 5% of patients in any treatment group were: diarrhoea, nausea, headache and upper respiratory tract infections.
Discontinuation due to gastrointestinal AEs was rare with apremilast 20 mg, 4.2% with apremilast 30 mg and 2.4% with placebo). Reported diarrhoea occurred in 51 patients on apremilast. Median onset was 9 days after starting the drug and median duration was 29.5 days. There were 59 events of nausea in 47 patients. Median onset was 10 days and median duration 17 days.
Few serious AEs occurred. There were 4 serious infections: 2 occurred on placebo and 2 in the apremilast 30 mg group . All four patients continued in the study. There were 2 myocardial infarctions , 2 malignancies . Overall, one patient died during the study . The cause of death was multi-organ failure secondary to pre-existing vitamin B12 deficiency and was considered unrelated to the study medication by the investigator. There were no case cases of tuberculosis , lymphoma or vasculitis.
There were few blood screening abnormalities detected. Of note, leucopaenia occurred in 0.6% with placebo, 2.6% on apremilast 20 mg and 1.3% with apremilast 30 mg.
Recommended Reading: How To Ease Arthritis Pain In Hands
Tips For Good Reviews
- Only rate drugs or treatments you’ve tried.
- In your description, mention the brand, dose, and period of time that you used the drug or treatment.
- Please share your positive and negative experiences with the drug, and compare it with other treatments you have used.
- Do not include any personal information or links in your review.
Otezla And Other Medications

Below is a list of medications that can interact with Otezla. This list doesnt contain all drugs that may interact with Otezla.
Different drug interactions can cause different effects. For instance, some can interfere with how well a drug works, while others can cause increased side effects.
Before taking Otezla, be sure to tell your doctor and pharmacist about all prescription, over-the-counter, and other drugs you take. Also tell them about any vitamins, herbs, and supplements you use. Sharing this information can help you avoid potential interactions.
If you have questions about drug interactions that may affect you, ask your doctor or pharmacist.
Drug metabolism inducers
Several medications can make an enzyme called cytochrome P450 3A4 more active in your body. Taking these drugs with Otezla can cause your body to metabolize Otezla more quickly than usual. It can also make Otezla less effective.
Examples of these medications include:
- carbamazepine
- phenobarbital
Herbs and supplements can sometimes interact with medications.
St. Johns wort
St. Johns wort can make an enzyme called cytochrome P450 3A4 more active in your body. Because of this, taking St. Johns wort with Otezla can cause your body to get rid of Otezla more quickly than usual. This can make Otezla less effective.
Taking too much of this medication can increase your risk for serious side effects.
Recommended Reading: Coping With Rheumatoid Arthritis
Safety Data From Palace 4
PALACE 4 reported low levels of AEs and SAEs. Again, diarrhoea and nausea were the two most commonly reported side effects. In the first year of therapy, these were reported in 10.3% of those on apremilast 20 mg and 10.9% of those on 30 mg. For those continuing in the trial for a second year, the rates were very low in year 2 . In PALACE 4, discontinuation due to AEs was rare. With apremilast 20 mg 5.6% in year 1, 1.7% in year 2. With apremilast 30 mg 5.2% in year 1, 3.5% in year 2
What Are The Side Effects Of Otezla
The most common side effects reported were diarrhoea and nausea. These side effects were mostly mild or moderate, and most common in the first two weeks settling down within four weeks. Other side effects reported commonly include upper respiratory tract infections, cough, vomiting, loss of appetite, headaches and fatigue . Most side effects were considered to be mild or moderate in severity.
In the clinical trials, some people reported experiencing depression. Before starting Otezla, tell your doctor if you have had feelings of depression, suicidal thoughts, suicidal behaviour, or other mood changes. After starting Otezla, tell your doctor if any of these symptoms develop or worsen.
Otezla is a new treatment and, as such, this side effect data comes from clinical trials.
Recommended Reading: Ra Symptoms In Hands
What About Side Effects
Some patients taking Otezla reported depression. Before you start Otezla, tell your doctor if you have had feelings of overwhelming sadness, thoughts or behaviors like wanting to killing yourself or, or other mood changes. After starting Otezla, tell your doctor if any of these symptoms develop or worsen.
The most common side effects of Otezla were diarrhea, headache, and nausea. Side effects usually occur within the first 2 weeks of treatment, and get better as you continue to take it. You may use over-the-counter medication such as immodium to treat diarrhea, or Tylenol® to treat headaches.
Tell your doctor about any side effect that bothers you or does not go away.
Is Otezla Effective
Otezlas new approval came after Amgen reported positive results from its phase 3 ADVANCE trial, which measured safety and effectiveness of the drug in people with mild or moderate psoriasis. There have been a few key findings from this trial so far:
- People who took apremilast were five times more likely to have less severe disease after four months of therapy, compared to people who took a placebo .
- Those who used apremilast were more likely to have smaller areas of their skin affected by psoriasis plaques and were less likely to have an affected scalp.
- People using apremilast had improved symptoms, such as less itching.
This trial also showed that apremilast is safe. Researchers identified a few potential side effects, including headache, nausea, diarrhea, and cold symptoms.
Also Check: How To Get Rid Of Arthritis Pain In Knees
How Do I Take It
When you first start Otezla, your doctor will give you a 14-day Starter Pack. All of the instructions are printed on the pack. During the first 5 days you gradually increase your dose until you reach the 30 mg twice a day. People with severe kidney disease will follow a different schedule for increasing their dose. Be sure to take Otezla exactly as directed by your doctor.
Otezla can be taken with or without food. Do not crush, chew or split the pill.
Otezla For Mouth Ulcers Caused By Behets Disease
Otezla is approved to treat mouth ulcers that occur with Behçets disease.
Behçets disease is an autoimmune disease. It causes damage to certain blood vessels that can lead to sores in your mouth, rashes, and other symptoms.
Effectiveness for Behçets disease
In a clinical study, 53% of people who took Otezla had their mouth ulcers completely cleared after 12 weeks. In comparison, 22% of people taking a placebo had that same result.
For 30% of people who took Otezla for 6 weeks, their mouth ulcers completely cleared and stayed cleared for another 6 weeks. In comparison, 5% of people taking a placebo had the same result.
Also, during the study, people taking Otezla had an average of 1.5 mouth ulcers. People taking the placebo had an average of 2.6 mouth ulcers.
Don’t Miss: Symptoms Of Arthritis In Knees And Legs
Ive Heard That Otezla Causes A Lot Of Nausea And Vomiting How Can I Prevent This
Yes, many people who take Otezla can have some nausea or vomiting. This is most likely to occur in the first 2 weeks of taking the medication. For most people, its not severe, and it often goes away with continued use of the drug.
To prevent nausea and vomiting, your doctor may need to lower your dosage. If your nausea doesnt go away or becomes severe, talk with your doctor. If lowering the dose doesnt help, you may need to stop taking Otezla.
Otezla is typically taken twice daily: once in the morning and once in the evening. For some people, such as those with kidney problems, it may be taken just once per day, in the morning.
Otezla can be taken on an empty stomach or with food.
Otezla tablets should be swallowed whole. They shouldnt be crushed, split, or chewed.
Who Is Otezla For
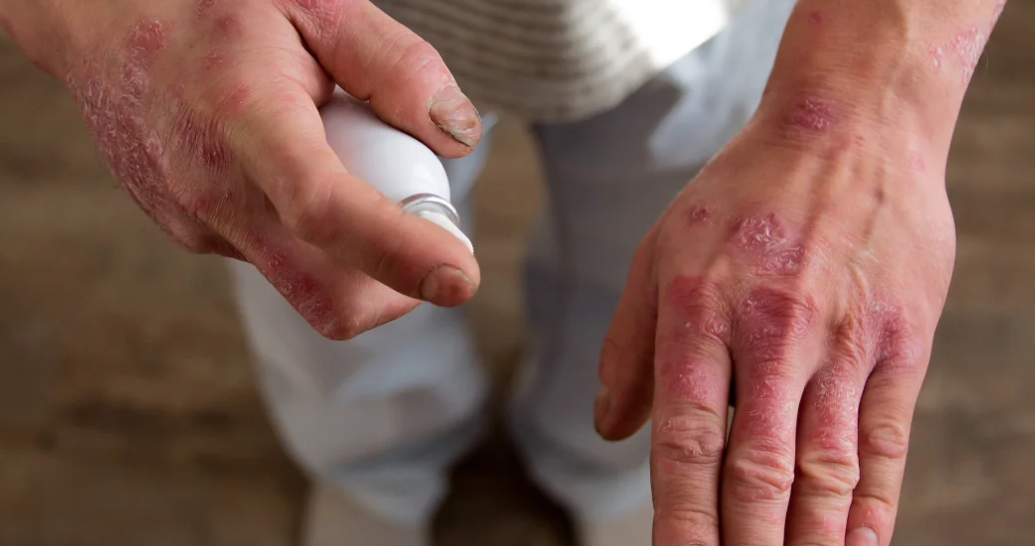
Otezla is intended for use in adults with moderate to severe chronic plaque psoriasis who have not responded to, or cannot take other systemic treatments including ciclosporin, methotrexate or PUVA for health reasons.
In Scotland, Otezla can also be used alone or in conjunction with another Disease-Modifying Anti-Rheumatic Drug , for example methotrexate, sulfasalazine or leflunamide, in adults with active psoriatic arthritis, who have not had an acceptable response to another DMARD, or who cannot take another DMARD for health reasons.
In England and Wales, Otezla can be used alone or in combination with other DMARDs in adults with active psoriatic arthritis who:
- Have peripheral arthritis with three or more swollen joints and three or more tender joints
- Have not had an acceptable response to at least two other DMARDs
Recommended Reading: Does Rheumatoid Arthritis Cause A Rash
Moderate To Severe Plaque Psoriasis
As defined in Aetna commercial policies, health care services are not medically necessary when they are more costly than alternative services that are at least as likely to produce equivalent therapeutic or diagnostic results. Avsola , Cimzia , Cosentyx , Enbrel , Remicade , Renflexis , and Siliq are more costly to Aetna than other targeted immune modulators for moderate to severe plaque psoriasis. There is a lack of reliable evidence that Avsola , Cimzia , Cosentyx , Enbrel , Remicade , Renflexis , and Siliq are superior to the lower cost targeted immune modulators: Humira, Ilumya, Inflectra, Otezla, Skyrizi, Stelara, Taltz, and Tremfya for moderate to severe plaque psoriasis. Therefore, Aetna considers Avsola , Cimzia , Cosentyx , Enbrel , Remicade , Renflexis , and Siliq to be medically necessary only for members who have a contraindication, intolerance or ineffective response to the available equivalent alternative targeted immune modulators: Humira, Ilumya, Inflectra, Otezla, Skyrizi, Stelara, Taltz, and Tremfya per criteria below. Requirements for a trial of lower cost targeted immune modulators are waived for persons requesting Cimzia who are pregnant or breastfeeding.
Criteria For Initial Approval
Moderate to severe plaque psoriasis
Aetna considers targeted immune modulators adalimumab , brodalumab , certolizumab , etanercept , guselkumab infliximab , ixekizumab , risankizumab-rzaa , secukinumab , tildrakizumab-asmn , or ustekinumab medically necessary for the treatment of moderate-to-severe plaque psoriasis who meet the following:
Active psoriatic arthritis
Aetna considers targeted immune modulators abatacept , adalimumab , certolizumab , etanercept , guselkumab , infliximab , ixekizumab , golimumab , secukinumab , and ustekinumab medically necessary for the treatment of active psoriatic arthritis .
Aetna considers all other indications as experimental and investigational .
Recommended Reading: What Can I Do For Arthritis In My Hands