Learn About All Your Plaque Psoriasis Treatment Options
With so many medicines available for the treatment of moderate-to-severe plaque psoriasis, you may be wondering which approach is best. Make sure to talk with your dermatologist about your treatment options, and ask if ILUMYA® can give you the long-lasting results youve been waiting for.
Topical medicines are treatments applied directly to your skin. Creams and ointments are examples of topical medicines. Topical treatments are usually one of the first medicines people try after they are diagnosed with plaque psoriasis.
Oral treatments are prescription pills taken by mouth. Oral medications are also called systemic treatments because they work throughout the body to fight plaque psoriasis.
Some biologics called TNF-alpha inhibitors block the TNF-alpha molecule that triggers inflammation in your body. In plaque psoriasis, too much TNF-alpha is produced in the skin. This leads to the rapid growth of skin cells. Biologic medicines that block TNF-alpha help stop the inflammation in psoriasis.
There are also other types of biologics for psoriasis.
ILUMYA® is an IL-23 blocker, an advanced biologic medicine used for moderate-to-severe plaque psoriasis treatment to help balance or regulate an overactive immune system.
ILUMYA® is different from topicals and pills. It works within your body to help reduce the redness, flaking, and scales you see on your skin.
COMPLETE THE FORM BELOW TO GET YOUR FREE COPY!
Please refer to our Privacy Policy for more information.
Aad Elects New Officers Board
The American Academy of Dermatology named its new officers and board members. These officers and board members will also hold the same position for the American Academy of Dermatology Association, a sister organization to the AAD that focuses on government affairs, health policy, and practice information.
Bruce H. Thiers, MD, FAAD, will be installed as president-elect in March 2019 and hold the office of president for one year beginning in March 2020. He is a distinguished university professor in the Medical University of South Carolina Department of Dermatology and Dermatologic Surgery in Charleston, SC.
Susan C. Taylor, MD, FAAD, will be installed as vice president-elect in March 2019 and hold the office of vice president for one year beginning in March 2020. She is an associate professor of dermatology at the University of Pennsylvania in Philadelphia.
Larry Green, MD, FAAD Adelaide Hebert, MD, FAAD Alexander Miller, MD, FAAD and Cyndi Yag-Howard, MD, FAAD, were elected to the Academy’s board of directors. They will each serve a four-year term beginning in March 2019.
Learn About Ilumya And Why Biologic Treatments May Be Right For You
Watch this webinar to hear Dr. Neal Bhatia, Director and Clinical Dermatology Principal Investigator at Therapeutics Clinical Research, discuss moderate-to-severe plaque psoriasis, the benefits of biologic treatments, and provide an overview of ILUMYA® as a treatment option. We also hear from Jim, one of Dr. Bhatiaâs patients currently taking ILUMYA®, who shares his journey with moderate-to-severe plaque psoriasis and how he found a biologic treatment that works for him. Dr. Bhatia answers some frequently asked questions and shares some new research in the field of biologics.
ILUMYA® is a prescription medicine used to treat adults with moderate to severe plaque psoriasis who may benefit from taking injections, pills, or phototherapy. Healthcare providers should check the patient for infections and tuberculosis before starting treatment with ILUMYA®. Before starting any treatment, patients should talk to their doctor and review the important safety information to understand the potential risks. The most common adverse reactions associated with ILUMYA® are upper respiratory infections, injection site reactions, and diarrhea.
This webinar is sponsored by Sun Pharma. For Important Safety Information: .
You May Like: Ra Symptoms In Hands
How Long Does It Take To Work
Ilumya will start to work as soon as you start taking it. However, it takes time to build up in your system and take full effect, so it may be several weeks before you see any results.
In clinical studies , after one week of treatment, fewer than 20 percent of people taking Ilumya saw an improvement in plaques. However, after 12 weeks, more than half of people who received Ilumya saw a significant improvement in their psoriasis symptoms. The number of people with improved symptoms continued to increase through 28 weeks of treatment.
What Is Plaque Psoriasis
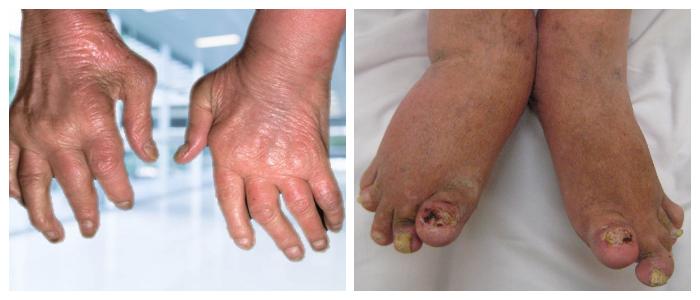
While no one knows the exact cause of plaque psoriasis, scientists do know that it starts in your immune system. Which is exactly why you need a treatment that moves past a surface-level approach and treats your symptoms at the source.
In plaque psoriasis, your skin cells grow more rapidly than normal, rising to the surface of your skin in days rather than weeks. This causes the plaques, redness, and flakes you see on your skin.
Read Also: 10 Rheumatoid Factor
Drug Forms And Administration
Ilumya comes in a single-dose prefilled syringe that contains 100 mg of tildrakizumab. Ilumya is given as an injection under the skin at the doctors office. The first two injections are given four weeks apart. After those injections, the doses are given every 12 weeks.
Like Ilumya, Tremfya comes in a single-dose prefilled syringe, but it contains 100 mg of guselkumab. Its also given as a subcutaneous injection. And as with Ilumya, the first two injections are given four weeks apart. However, all doses after that are given every eight weeks.
Tremfya can be given at your doctors office, or self-injected at home after you receive proper training from your healthcare provider.
Uses That Arent Approved
Ilumya may be used off-label for other conditions. Off-label use is when a drug thats approved to treat one condition is prescribed to treat a different condition.
Psoriatic arthritis
Ilumya isnt approved to treat psoriatic arthritis, but it may be prescribed off-label for this condition. Psoriatic arthritis involves psoriasis symptoms of the skin as well as sore, swollen joints.
In one small clinical study, Ilumya didnt significantly improve psoriatic arthritis symptoms or pain when used for 16 weeks, compared to a placebo .
However, additional studies are being done to test whether Ilumya is useful in treating psoriatic arthritis. Another long-term clinical study is currently ongoing.
Ankylosing spondylitis
Ilumya isnt approved for the treatment of ankylosing spondylitis . However, there is an ongoing clinical study to test whether its effective for this condition.
The following information describes the usual dosage for Ilumya. However, be sure to take the dosage your doctor prescribes for you. Your doctor will determine the best dosage to suit your needs.
Don’t Miss: Vicks Vaporub For Joint Pain
Why Do Dermatologists Prescribe A Biologic To Treat Psoriasis
A biologic is an important treatment option for people with moderate-to-severe psoriasis, psoriatic arthritis, or both. For many people, taking a biologic was life changing because it helped control their symptoms when other treatments failed.
Benefits of biologics
Using a biologic to treat psoriasis is life changing for some people.
Biologics work by blocking reactions in your body that cause psoriasis and its symptoms.If you have psoriatic arthritis, a biologic can stop the pain, stiffness, and swelling in your joints. It can prevent the arthritis from worsening and causing more damage to your joints. The US Food and Drug Administration has approved the following biologics to treat adults with psoriasis or psoriatic arthritis. In many cases, these biologics have been approved to treat both diseases.
| Drug |
|---|
| X |
1Approved to treat adults when other psoriasis treatments fail to work or stop working.
Sometimes, a biologic is prescribed to treat a child who has psoriasis. This can be very effective for a child who has severe psoriasis. The FDA has approved usetekinumab to treat people 12 years of age and older who have moderate-to-severe psoriasis.
Fda Approves Ilumya For Plaque Psoriasis
Clinical trial data show drug may help achieve clear skin.
Adults with moderate-to-severe plaque psoriasis will soon have access to a new treatment option. On March 21, 2018, the U.S. Food and Drug Administration approved Ilumya for adults who are eligible for systemic therapy or phototherapy.
Dosage calls for an injection of 100 mg every 12 weeks, after two initial injections at weeks 0 and 4. Ilumya works by binding to interleukin-23, a key protein involved in inflammatory diseases.
âAs we move to finding a cure for psoriasis, what we are looking for are high performance drugs that provide durable response in between treatments and that lend themselves to patient compliance â in this case 12 weeks. This lessens the need for multiple injections along the way,â says dermatologist George Martin, M.D., a member of NPFâs Medical Board. âItâs exciting that we have newer, very powerful choices.â
Results from two Phase 3 clinical trials showed significant improvement in patients who received Ilumya compared with the placebo. In one study, 74 percent achieved 75 percent skin clearance at week 28 after three doses. Meanwhile, 84 percent who continued receiving Ilumya maintained 75 percent skin clearance at week 64, compared with 22 percent who were randomly reassigned to placebo.
Read Also: Acute Arthritis Attack
Ilumya Use With Other Drugs
Ilumya is effective at improving plaque psoriasis on its own, but it can also be used with other medications for additional benefit. Using more than one method to treat psoriasis can help clear plaques faster and clear a greater percentage of plaques.
Combination therapy can also decrease the dosage you need of other psoriasis medications, which decreases your risk of side effects. In addition, combination therapy may reduce your risk of developing resistance to Ilumya .
Examples of other therapies that can be safely used with Ilumya include:
- topical corticosteroids, such as betamethasone
- topical vitamin D creams and ointments
- methotrexate
Is Ilumya Used To Treat Psoriatic Arthritis
Ilumya is not FDA-approved to treat psoriatic arthritis, but it may be used off-label for that purpose.
In one small clinical study, Ilumya didnt significantly improve psoriatic arthritis symptoms or pain, but additional studies are being done to test whether its useful for this condition. Another long-term clinical study is currently ongoing.
Recommended Reading: What Rheumatoid Arthritis Feels Like
Before Receiving This Medicine
You should not be treated with Ilumya if you are allergic to tildrakizumab
Tell your doctor if you have a chronic infection, or if you are scheduled to receive any vaccine.
Tell your doctor if you have ever had tuberculosis, or if anyone in your household has tuberculosis.
It is not known whether Ilumya will harm an unborn baby. Tell your doctor if you are pregnant or plan to become pregnant.
It may not be safe to breast-feed while using tildrakizumab. Ask your doctor about any risk.
Ilumya is not approved for use by anyone younger than 18 years old.
I Have Always Used Creams For My Plaque Psoriasis Why Do I Need To Start Receiving Injections
Your doctor may have decided that a systemic treatment could do more to relieve your symptoms than your creams. Systemic drugs are given by injection or taken by mouth and work throughout the whole body.
Systemic treatments such as Ilumya are generally more effective at improving psoriasis symptoms than topical treatments . This is because they work from the inside out. They target the immune system itself, which causes your psoriasis plaques. This can help both clear and prevent psoriasis plaques.
Topical treatments, on the other hand, generally treat the plaques after theyre formed.
Systemic treatments are sometimes used in combination with, or instead of, topical treatments. They may be used if:
- topical medications dont improve your plaque psoriasis symptoms enough, or
- plaques cover a large portion of your skin , making topical treatments impractical. This is considered moderate to severe psoriasis.
Read Also: Arthritis In Fingers Remedy
Hydrogen Peroxide 40% Less Cytotoxic Than Cryotherapy: Study
Hydrogen peroxide topical solution 40% is less cytotoxic to living cells and less damaging to melanocytes than cryosurgery, according to a new study led by Adam Friedman, MD, associate professor of dermatology at the GW School of Medicine and Health Sciences.
The study evaluated skin architecture, metabolic activity, and cytotoxicity, with a particular emphasis on melanocytes, utilizing a validated ex-vivo human reconstituted full thickness model derived from Fitzpatrick V skin .
There is ongoing clinical work evaluating the safety of this solution in darker skin types, explains Dr. Friedman in a statement. Another important take away from this study is to also recognize how damaging even a small amount of cryotherapy, a five-second free time, can be to human skin. These findings should be considered when using for the broad array of skin diseases for which cryosurgery is warranted.
Findings appear in the Journal of the American Academy of Dermatology.
Side Effects Not Requiring Immediate Medical Attention
Some side effects of tildrakizumab may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects.
Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
Less common
- Bleeding, blistering, burning, coldness, discoloration of skin, feeling of pressure, hives, infection, inflammation, itching, lumps, numbness, pain, rash, redness, scarring, soreness, stinging, swelling, tenderness, tingling, ulceration, or warmth at the injection site
Read Also: High Rheumatoid Factor Causes
What To Discuss With Your Dermatologist
You should tell your dermatologist if you:
-
Have any side effects while taking the biologic
-
Stop taking the biologic
-
Have questions, including how to take the biologic
-
Become pregnant
Biologics and vaccines
Before you get a flu shot or vaccinated against any disease, call your dermatologist. You should NOT get some vaccines while taking a biologic.
Related AAD resources
Cordoro KM. Management of childhood psoriasis. Adv Dermatol. 2008 24:125-69.
Feldman SR. Treatment of psoriasis. UpToDate 2015 Jul, Wolters Kluwer Health. Last accessed November 2015.
Kim WB, Marinas JEC, et al. Adverse events resulting in withdrawal of biologic therapy for psoriasis in real-world clinical practice: A Canadian multicenter retrospective study. J Am Acad Dermatol 2015 73:237-41.
Motaparthi K, Stanisic V, et al. From the Medical Board of the National Psoriasis Foundation: Recommendations for screening for hepatitis B infection prior to initiating antietumor necrosis factor-alfa inhibitors or other immunosuppressive agents in patients with psoriasis. J Am Acad Dermatol. 2014 Jan 70:178-86.
Singh JA, Wells GA, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No.: CD008794. DOI: 10.1002/14651858.CD008794.pub2.
All content solely developed by the American Academy of Dermatology
The American Academy of Dermatology gratefully acknowledges the support from Amgen and .
Full Important Safety Information & Indications
Important Safety Information You Should Know About CIMZIA® Serious and sometimes fatal side effects have been reported with CIMZIA, including tuberculosis , bacterial sepsis, invasive fungal infections , and infections due to other opportunistic pathogens . Patients should be closely monitored for the signs and symptoms of infection during and after treatment with CIMZIA. Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, of which CIMZIA is a member. CIMZIA is not indicated for use in pediatric patients. to open Full Prescribing Information.
Also Check: Arthritis Remission
Whats A Biologic Drug
A biologic drug is a medication thats created from human or animal proteins. Biologic drugs used to treat autoimmune diseases, such as plaque psoriasis, work by interacting with the bodys immune system. They do this in targeted ways to reduce inflammation and other symptoms of an overactive immune system.
Because they interact with very specific immune system cells and proteins, biologics are thought to have fewer side effects compared to drugs that affect a wider range of body systems, as many drugs do.
When used to treat psoriasis, biologic drugs are generally used for people with moderate to severe plaque psoriasis who dont respond to other treatments .
Before Starting Ilumya Treatment
Because Ilumya weakens your immune system, your doctor will check you for tuberculosis before you start treatment. If you have active TB, youll receive TB treatment before starting Ilumya. And if you had TB in the past, you may need to be treated before starting Ilumya.
But even if you dont have TB symptoms, you could have an inactive form of TB, which is called latent TB. If you have latent TB and take Ilumya, your TB could become active. If the testing shows that you have latent TB, youll likely need to receive TB treatment before or during your treatment with Ilumya.
Read Also: How To Prevent Rheumatoid Arthritis In Hands
Sun Pharmas Ilumya Improves Joint And Skin Symptoms Of Psoriatic Arthritis
14 June 2019 | News
Over 71% of Patients Treated with ILUMYA Achieved ACR20 Response After 24 Weeks, with Significant Improvement as Early as 8 Weeks
Sun Pharmaceutical Industries Ltd. recently announced interim results from a Phase 2 study of interleukin-23 inhibitor ILUMYA in patients with active psoriatic arthritis that was presented in a late-breaking oral presentation at the Annual European Congress of Rheumatology in Madrid, Spain.
The interim analysis revealed that over 71 percent of patients treated with ILUMYA experienced a 20 percent improvement in joint and skin symptoms , meeting the primary endpoint of the study. The interim results showed ILUMYA was well tolerated with a low rate of serious treatment-emergent adverse events.
ILUMYA is approved in the U.S. for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy and is being investigated for psoriatic arthritis. Psoriatic arthritis, which affects up to 42 percent of people with plaque psoriasis, is an inflammatory condition that impacts both the joints and skin. It is painful, causes fatigue, and can lead to swelling and stiffness of the joints that may reduce range of motion. If left untreated, this chronic condition can lead to permanent joint damage.
Sign up for the editor pick and get articles like this delivered right to your inbox.
L’oral And Skinceuticals Unveil Custom Dose
Developed by L’Oréal’s Technology Incubator in partnership with L’Oréal-owned SkinCeuticals, CUSTOM D.O.S.E is a new service engineered to scan and evaluate consumers’ unique skin needs and combine active ingredients into a tailor-made, corrective serum.
The D.O.S.E technology is able to mix active ingredients, chosen specifically to target the appearance of skin aging issues, like wrinkles, fine lines, and discoloration, into a single serum. D.O.S.E has a production-quality compounder that operates at 1,200 rotations per minute to mix ingredients precisely, drop by drop. The machine combines active ingredients that historically were unmixable outside of a factory setting. Through this technology, skincare professionals can administer a single D.O.S.E serum with multiple active ingredients that address the appearance of multiple skin concerns. More than 250 unique skin types were considered when researching and selecting active ingredients to include in D.O.S.E in order to provide dozens of combinations through over 2,000 algorithms.
The D.O.S.E experience begins with a one-on-one skincare professional consultation to discuss which active ingredients will be most appropriate for the patient. The skincare professional completes an assessment on a tablet, which transfers the data to the D.O.S.E machine that mixes and dispenses the customized serum. A custom label is then printed for each consumer, including an expiration date and a bar code for easy reordering.
Don’t Miss: Can You Get Arthritis In Your Legs